Andrew L. Sargent
Professor,
Inorganic and Theoretical Chemistry
- Army High Performance Computing Research Center (1992-1994)
- Postdoctoral Fellow, Minnesota Supercomputer Institute (1991-1992)
- Ph.D., Texas A&M University (1991)
- B.A., The Colorado College (1985)
Research Overview
Many of the projects we undertake involve the investigation of catalytic organometallic reactions. Unique to our approach is the use of the Nudged Elastic Band (NEB) method (Alfonso, D.R.; Jordan, K.D. J. Comput. Chem. 2003, 24, 990-996) to follow the complex reaction energy path (REP). In this method, a series of interpolated structures, called images, is generated to depict the geometric evolution between two stationary points, and the corresponding energies and energy gradients are computed for each image. The total gradient, or force, on each image, is projected into so-called parallel and perpendicular forces, where the former refers to the force along the REP, while the latter is that normal to the REP. Force constants are applied along the parallel direction to prevent the images from collapsing back to the local minima (the reactant or product) and to keep the images evenly spaced. The perpendicular forces are quantum mechanically minimized in mass weighted coordinates to accelerate convergence, which is particularly useful for systems with heavy atoms. Although the climbing image option of the NEB method can, in theory, be run to convergence to obtain the transition state (TS), in practice this is rarely done. Instead, selected data from the NEB run is fed to the Dimer method (Henkelman, G.; Jónsson, J. J. Chem. Phys.1999, 111, 7010-7022; Heyden, A.; Bell, A.T.; Keil, F.J. J. Chem. Phys. 2005, 123, 224101-22414) to locate the TS.
Application of the NEB method is resource intensive, but our in-house version of the program (thanks to Dr. Lee Bartolotti) allows us to take advantage of large cluster computing platforms to run calculations in parallel. Not only do we evaluate the typical components of an electronic structure calculation in parallel, but within a given NEB cycle the evaluation of each image is independent of the others, so we apply an additional layer of parallelism to the simultaneous evaluation of images in what we refer to as our “parallel-parallel” approach.
In practice, the NEB approach has helped us locate unexpected minima and map paths involving asynchronous atomic movement, all with the guarantee that the TS is connected to the local minima. In cases where more than one pathway exists between endpoints, NEB distinguishes between the paths and is able to locate the one of lowest energy. This is illustrated simply in the left-hand portion of Scheme 1. Even though TS 1 is a transition state on a REP between the two endpoints, if it is not the minimum energy path, the perpendicular forces will move the images in a direction away from it. Even if an unidentified intermediate (INT 1) exists, the perpendicular forces will pull the REP towards the intermediate and two transition states will be located.
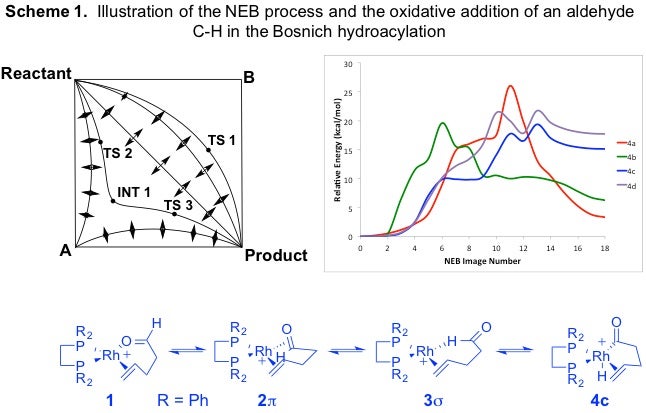
For example, our recent re-examination of the oxidative addition (OA) step in the classic Bosnich rhodium-catalyzed hydroacylation reaction of 4-pentenal (Fairlie, D.P.; Bosnich, B. Organometallics 1988, 7, 946-954) revealed that the C-H cleavage step was more complex than the putative single transition state connecting the two local minima originally proposed. NEB calculations that used 19 images for the oxidative addition reactions leading to the four possible isomers (hydride/acyl, equatorial/apical-up or –down in square pyramidal Rh(III) complex) are shown on the right in Scheme 1. Careful analysis revealed that the lowest energy pathway leading to cyclopentanone product evolved through two additional local minima (carbonyl pi and C-H sigma complexes shown below) and proceeded through one of the higher-energy OA isomer intermediates. The transition state originally identified using traditional QST methods was higher in energy and did not exist along the lower energy NEB reaction energy path. The fine structure of the NEB REPs helped us identify additional local minima that are important not only from the perspective of constructing an accurate reaction mechanism, but also from a catalysis and ligand-design perspective.
Selected Publications
“Alternate Geometries in the Cobalt-Catalyzed Hydroacylation of Dienes Facilitate a High Spin Mechanism: A DFT Study,” T.E. Shoopman, A.T. Morehead Jr., A.L. Sargent, Organometallics, 2024, 43, 2202-2212.
“Association between N-Terminal Pyrenes Stabilizes the Collagen Triple Helix,” J.M. Keever, P.D. Banzon, M.K. Hales, A.L. Sargent, W.E. Allen, J. Org. Chem. 2023, 88, 11885-11894.
“Synthesis of Redox-Active Fluorinated 5-Hydroxytryptophans as Molecular Reporters for Biological Electron Transfer,” A. Ohler, H. Long, K. Ohgo, K. Tyson, D. Murray, A. Davis, C. Whittington, E.G. Hvastkovs, L. Duffy, A. Haddy, A.L. Sargent, W.E. Allen, A.R. Offenbacher, Chem. Comm. 2021, 57, 3107-3110.
“Side-Chain Protonation States of a Fluorescent Arginine,” S.R. Marshall, M.L. Stoudt, M.A. DiVittorio, A.L. Sargent, W.E. Allen, J. Org. Chem. 2019, 84, 14407-14413.
“DFT Mechanistic Investigation of an Enantioselective Tsuji-Trost Allylation Reaction,” K.E. McPherson, M.P. Croatt, A.T. Morehead Jr., A.L. Sargent, Organometallics 2018, 37, 3791-3802.
“Decarboxylative and Dehydrative Coupling of Dienoic Acids and Pentadienyl Alcohols to form 1,3,6,8-Tetraenes,” G. I. A. Deiab, M. H. Al-Huniti, I. F. D. Hyatt, E. E. Nagy, K. E. Gettys, S. S. Sayed, C. M. Joliat, P. E. Daniel, R. M. Vummalaneni, A. T. Morehead Jr., A. L. Sargent, M. P. Croatt, Beilstein J. Org. Chem. 2017, 13, 384-392.
“Utility of the Nudged Elastic Band Method in Identifying the Minimum Energy Path of an Elementary Organometallic Reaction Step,” K. E. McPherson, L. J. Bartolotti, A. T. Morehead, A. L. Sargent, Organometallics. 2016, 35, 1861-1865.
“Incorporation of fluorophore-cholesterol conjugates into liposomal and mycobacterial membranes,” A. N. Wercholuk, J. M. Thuman, J. L. Stanley, A. L. Sargent, E. S. Anderson, W. E. Allen, Bioorg. Med. Chem. 2016, 24, 1045-1049.
“Anion binding by fluorescent Fmoc-protected amino acids,” D. P. Farrell, A. L. Sargent, W. E. Allen, Supramol. Chem. 2016, 28, 45-52.
“Dimerization and Anion Binding of a Fluorescent Phospholipid Analogue,” D. M. Jessen, A. N. Wercholuk, B. Xiong, A. L. Sargent, W. E. Allen, J. Org. Chem. 2012, 77, 6615-6619.
“Binding of Carboxylic Acids by Fluorescent Pyridyl Ureas,” L.M. Jordan, P.D. Doyle, A.L. Sargent, W.E. Allen, J. Org. Chem. 2010, 75, 8450-8456.
“Wurster-Type’ Ureas as Redox-Active Receptors for Anions,” J. P. Clare, A. Statnikov, V. Lynch, A. L. Sargent, J. W. Sibert, J. Org. Chem. 2009, 74, 6637-6646.
“Fluorous Effects in Amide-Based Receptors for Anions,” J. V. Gavette, J. M. McGrath, A. M. Spuches, A. L. Sargent, W. E. Allen, J. Org. Chem. 2009, 74, 3706-3710.
“Hydrogen Bonding vs Steric Gearing in a Hexasubstituted Benzene,” J. V. Gavette, A. L. Sargent, W. E. Allen J. Org. Chem. 2008, 73, 3582-3584.
“Mechanism of Rhodium-Catalyzed Intramolecular Hydroacylation: A Computational Study,” I. F. D. Hyatt, H. K. Anderson, A. T. Morehead Jr., A. L. Sargent, Organometallics 2008, 27, 135-147.
“On the Oxidation of Wurster’s Reagent and the Wurster’s Crown Analog of 15-Crown-5 in the Presence of Alkali Metal Cations,” M. DeBacker, M. Hureau, M. Depriester, A. Deletoille, A. L. Sargent, P. B. Forshee, J. W. Sibert, J. Electroanal. Chem. 2008, 612, 97-104.
“Wurster’s Crowns: A Comparative Study of ortho- and para-Phenylenediamine-Containing Macrocyclic Receptors,” J. W. Sibert, P. B. Forshee, G. R. Hundt, A. L. Sargent, S. G. Bott, V. Lynch, Inorg. Chem. 2007, 46, 10913-10925.
“An Interactive Computer Program to Help Students Learn Molecular Symmetry Elements and Operations,” D. E. Meyer, A. L. Sargent, J. Chem. Educ. 2007, 84, 1551-1552.
“A Theoretical Investigation on the Wurster’s Crown Analogue of 18-Crown-6,” A. L. Sargent, B. J. Mosley, J. W. Sibert, J. Phys. Chem. A 2006, 110/10, 3826-3837.
“Wurster’s Crownophanes: An Alternate Topology for para-Phenylenediamine-Based Macrocycles,” J. W. Sibert, G. R. Hundt, A. L. Sargent, V. Lynch, Tetrahedron 2005, 61/52, 12350-12357.
“Participation of Host ‘Spacer’ Atoms in Carboxylic Acid Binding: Implications for Amino Acid Recognition,” D. K. Barnhill, A. L. Sargent, W. E. Allen, Tetrahedron 2005, 61/35, 8366-8371.
“Dynamic Disorder and Conformer Exchange in the Crystalline Monomer of Polycarbonate,” J. E. Wolak, J. Knutson, J. D. Martin, P. Boyle, A. L. Sargent, J. L. White, J. Phys Chem. B 2003, 107, 13293-13299.
“Global vs. Local Aromaticity in Porphyrinoid Macrocycles: Experimental and Theoretical Study of ‘Imidacene,’ an Imidazole-Containing Analogue of Porphycene,” A. L. Sargent, I. C. Hawkins, W. E. Allen, H. Liu, J. L. Sessler, C. J. Fowler, Chem. Eur. J. 2003, 9, 3065-3072.
“C-S and C-H Bond Activation of Thiophene by Cp*Rh(PMe3): A DFT Theoretical Investigation,” A. L. Sargent and E. P. Titus, Organometallics, 1998, 17, 65-77.
“Electronic Structure of Axially Ligated Rhodium Carboxylates: pi Back-Bonding Revisited,” A. L. Sargent, M. E. Rollog and C. T. Eagle, Theor. Chem. Acc., 1997, 97, 283-288.
“Poly(Thienyl)Borates: An Investigation Into the Coordination of Thiophene and Its Derivatives,” A. L. Sargent, E. P. Titus, C. G. Riordan, A. L. Rheingold and P. Ge, Inorg. Chem., 1996, 35, 7095-7101.
“Enzyme-Catalyzed Enolization Reactions: A Theoretical Study on the Energetics of Concerted and Stepwise Pathways,” A. L. Sargent, M. E. Rollog, J. Almlof, P. G. Gassman and J. Gerlt, J. Molec. Struct. (THEOCHEM), 1996, 388, 145-159.
“Massively Parallel Algorithms for Electronic Structure Calculations in Quantum Chemistry,” A. L. Sargent, J. Almlof and M. W. Feyereisen, in Applications on Advanced Architecture Computers, G. Astfalk, Ed., Vol. 1, 1996, 1-13.
“Electron Delocalization in Helical Bis(quinone) Anion Radicals,” A. L. Sargent, J. Almlof, and C. A. Liberko, J. Phys. Chem., 1994, 98, 6114-6117.
“Massively Parallel Algorithms for Electronic Structure Calculations in Quantum Chemistry,” A. L. Sargent, J. Almlof, and M. W. Feyereisen, SIAM News, 1993, 26(1), 14.
“Theoretical Studies of Inorganic and Organometallic Reaction Mechanisms. 4. The Oxidative Addition of Dihydrogen to d8 Square-Planar Iridium Complexes With Trans Phosphines,” A. L. Sargent and M. B. Hall, Inorg. Chem., 1992, 31, 317-321.
“Theoretical Studies of Inorganic and Organometallic Reaction Mechanisms. 3. The Origin of the Difference in the Barrier for the Kinetic and Thermodynamic Products for the Oxidative Addition of Dihydrogen to a Square-Planar Iridium Complex,” A. L. Sargent, M. B. Hall, and M. F. Guest, J. Am. Chem. Soc., 1992, 114, 517-522.
“The Oxidative Addition of H2 to d8 Square-Planar Iridium Complexes,” A. L. Sargent and M. B. Hall, in Topics in Physical Organometallic Chemistry, Vol. 4, M. Gielen, Ed., Freund Pub.: London, England, 1992; pp 1-39.
“Basis Sets for Geometry Optimizations of Second-Row Transition Metal Inorganic and Organometallic Complexes,” Sargent, A. L.; Hall, M. B. J. Comp. Chem. 1991, 12, 923‑933.
“Linear Semibridging Carbonyls – 3. Carbonyl and Thiocarbonyl Ligands as Four‑Electron Donors,” Sargent, A. L.; Hall, M. B. Polyhedron, 1990, 15/16, 1799‑1808.
“Linear Semibridging Carbonyls – 2. Heterobimetallic Complexes Containing a Coordinatively Unsaturated Late Transition Metal Center,” Sargent, A. L.; Hall, M. B. J. Am. Chem. Soc. 1989, 111, 1563‑1569.