East Carolina University Titration Wall of Fame
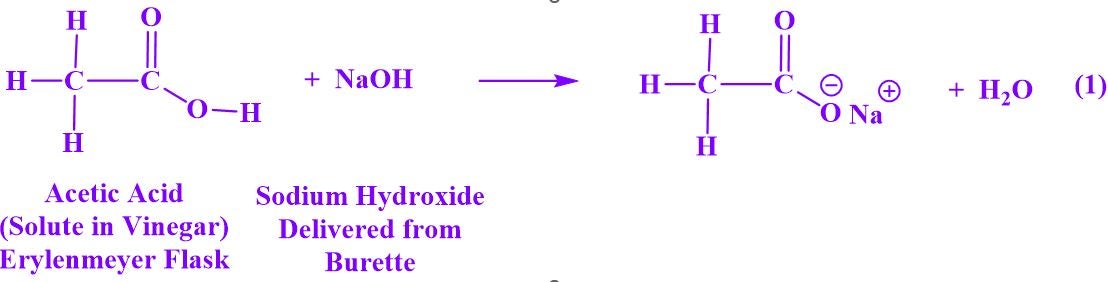
General Chemistry II students employed the principles of an Acid-Base Neutralization reaction and a titration to determine the concentration of acetic acid in commercial vinegar. In the titration experiment presented here, an alkaline solution of sodium hydroxide was delivered from a burette into the Erlenmeyer flask containing samples of a vinegar solution of acetic acid and an indicator, phenolphthalein that provides a visual observation for the end point of a titration (eq 1). Phenolphthalein turns various colors ranging from colorless in acidic solutions to vibrant purple under alkaline conditions. It is expected at the equivalence point that exact proportions of sodium hydroxide and acetic acid have been stoichiometrically combined resulting in a very faint pink colored solution that is barely detectable by the eye. Alternatively, if the amount of base exceeds what is required at the equivalence point, then a vibrant pink or purple color is detected (not desirable).
The CHEM 1161 students presented here from the Spring semester of 2024 have met the challenge of a careful titration and mastered the skill of “splitting a drop”. As many discovered, this task was not as trivial as it might first appear and required several attempts. They learned that with a little bit of patience, perseverance, and practice, they could isolate that “perfect faint pink color” required for a successful titration.


