Shouquan Huo

Associate Professor,
Inorganic and Organic Chemistry
Office: SZ 543
Phone: 252-328-9784
E-mail: huos@ecu.edu
- Senior Scientist (2003), Principal Scientist (2006), Eastman Kodak Company
- Senior Research Chemist, DSM Pharmaceuticals, Inc (2002
- Postdoctoral associate, Purdue University (1998)
- COE (Center of Excellence) Research Fellow, Hokkaido University, Japan (1996)
- Visiting Scientist, Institute for Molecular Sciences, Japan (1995)
- NNSF Postdoctoral fellow, Shanghai Institute of Organic Chemistry, China (1994)
- Ph.D. Chemistry, Nanjing University, China (1994)
- BS/MS Zhengzhou University, China (1988,1991)
Research in Dr. Huo’s Group
Our group is interested in the organometallic reactions and catalysis, synthesis and characterization of functional organometallic materials, and biological applications of organometallic compounds. Current research pursues in the following directions.
Organometallic C-H bond activation and functionalization. The control of selectivity in competing sp2/sp3 C-H bond activations is challenging, even with the intramolecular version of organometallic C-H bond activations. Our group discovered an interesting solvent-controlled switch of selectivity between sp2 and sp3 C-H bond activation by platinum (Chem. Commun. 2011, 47, 1902-1904). As shown in Scheme 1, the reaction of the bipyridine ligand with K2PtCl4 in acetic acid produced predominantly sp3 C-H activation products, while in acetonitrile (MeCN) gave selectively sp2 C-H activation products. sp2 C-H activation products can thermally isomerize to their corresponding sp3 C-H activation products in acetic acid. This is a classical example of thermodynamic and kinetic control of reactions.
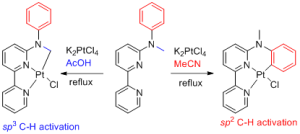
Scheme 1
During the study on the selective C-H bond activation, we discovered an unprecedent regiospecific acylation of cycloplatinated complexes in which the acylation occurs at the metalated carbon (Organometallics, 2016, 35, 1313-1322). More recently, this process has been developed into an efficient Pt-catalyzed C-H acylation reaction (Scheme 2) (Org. Lett. 2017, 19, 1606-1609). This reaction distinguish itself from other metal-catalyzed acylation reactions by the fact that no oxidant and other additives are needed and a broader range of acyl groups can be introduced. We are currently expanding our research in this area by investigating the scope, limitations, and mechanistic aspects of this reaction and other related reactions.

Scheme 2
Phosphorescent materials. Phosphorescent materials based on cyclometalated iridium and platinum compounds have been used as triplet emitters in organic light-emitting diode (OLED) devices to improve the devices’ efficiency through harvesting both singlet and triplet excited states generated by electric excitations. Our group has designed, synthesized, and characterized many highly efficient phosphorescent platinum complexes based on tridentate and tetradentate ligands (Scheme 3, Inorg. Chem. 2010, 49, 5107-5119). Some of the complexes are among the brightest Pt-based emitters. Through structural engineering of the complexes, emissions covering entire visible spectrum (blue to red) can be achieved. Our research in this area is focused on the structure-property relationship of phosphorescent materials and synthesis of tailor-made functional phosphorescent materials for optoelectronic and biological applications.

Scheme 3
Organometallic anticancer agents and imaging materials. In collaboration with Professor Yan-Hua Chen at the Brody School of Medicine, we have screened a series of phosphorescent cyclometalated platinum compounds for their anticancer activities. The cellular uptake of those luminescent complexes is clearly demonstrated by the cell imaging (Scheme 4). A significant finding is the drastic difference in cytotoxicity between isomeric platinum complexes (N^C^N)PtCl and (C^N^N)PtCl (J. Inorg. Biochem. 2014, 134, 49-56). The N^C^N-coordinated Pt complex demonstrated remarkably higher toxicity against a series of human lung cancer cells and a prostate cancer cell, probably attributed to the trans effect of the carbon donor in N^C^N-coordinated complex. The screening has led us to the investigation of NCN-coordinated complexes as anticancer agents and other low cytotoxic complexes as imaging and/or labeling materials in biological applications.
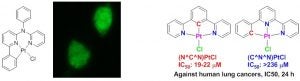
Scheme 4
Book and Book Chapters
- S. Huo and Yumin Li
Phosphorescent Materials
In The ACS In Focus Series, American Chemical Society, May, 2023. - S. Huo and Yumin Li
Pt-Catalyzed C-C Bond Formation through C-H Functionalization
In Handbook of C-H Functionalization, Maiti (Ed.). Wiley-VCH, October 1st, 2022, published online, https://doi.org/10.1002/9783527834242.chf0096. - S. Huo and Yumin Li
Pt-Catalyzed C-X (X=O, B, Si, Cl) Bond Formation through C-H Functionalization
In Handbook of C-H Functionalization, Maiti (Ed.). Wiley-VCH, October 2nd, 2022, published online, https://doi.org/10.1002/9783527834242.chf0095 - S. Huo
Carboalumination Reactions
In The Chemistry of Organoaluminum Compounds in PATAI’s Chemistry of Functional Groups, L. Micouin, I. Marek, Z. Rappoport and Eds, John Wiley and Sons, Chichester, May, 2017, pp. 253-316. - S. Huo and E. Negishi
Palladium-Catalyzed Alkenyl-Aryl, Aryl-Alkenyl, and Alkenyl-Alkenyl Couplings
In The Handbook of Organopalladium Chemistry for Organic Synthesis, E. Negishi, Ed., J. Wiley & Sons, New York, Sect. III.2.6, 2002, pp. 335-408. - E. Negishi and S. Huo
Synthesis and Reactivity of Zirconocene Derivatives
In Titanium and Zirconium in Organic Synthesis, Chapter 1, Marek, I., Ed., Wiley-VCH, 2002, pp. 1-49. - E. Negishi and S. Huo
(-)-dichlorobis[(1,2,3,3a,7a-e)-1-[(1S,2S,5R)-5-methyl-2-(1-methylethyl)]cyclohexyl-1H-inden-1-yl]zirconium and its (+)-(1R,2R,5S)-isomer
In e-EROS Electronic Encyclopedia of Reagents for Organic Synthesis, Paquette, L., Rigby, J., Roush, W., and Wipf, P., Ed., John Wiley & Sons, Inc., New York, 2001
Review Articles
- S. Huo
Platinum in Chemistry. An adventure from phosphorescent materials to catalytic C-H functionalization.
The Chemical Record, invited personal account, 2018, 18, 1583-1595. - S. Huo, J. Carroll, and D. A. K. Vezzu
Design, synthesis, and applications of highly phosphorescent cyclometalated platinum complexes
Asian J. Org. Chem. 2015, 4, 1210-1245. Focus Review. - S. Huo, R. Mroz, and J. Carroll
Negishi coupling in the synthesis of advanced electronic, optical, electrochemical, and magnetic materials
Org. Chem. Frontiers. 2015, 2, 416-445. - E. Negishi and S. Huo
Zirconium-catalyzed Enantioselective Carboalumination of “Unactivated” Alkenes as a New Synthetic Tool for Asymmetric Carbon-carbon Bond Formation
Pure Appl. Chem. 2002, 74, 151-157. - Y. Wu, S. Huo, J. Gong, X. Cui, L. Ding, K. Ding, C. Du, Y. Liu, and M. Song
Studies on Cyclometallation of Ferrocenylimines
J. Organomet. Chem. 2001, 637-639, 27-46. Invited review
Original Research Articles
- A. Barham, J. Neu, C. L. Canter, R. D. Pike, Y. Li, S. Huo*
Isomerization-Induced Multiple Reaction Pathways in Platinum-Catalyzed C−H Acylation Reaction of 2‑Aryloxypyridines.
Organometallics 2021, 40, 3158-3169.
- E. Warden, L. Bartolotti, S. Huo,* Y. Li*
Theoretical Probe to the Mechanism of Pt-Catalyzed C−H Acylation Reaction: Possible Pathways for the Acylation Reaction of a Platinacycle.
Inorg. Chem. 2020, 59, 555−562.
- E. Javed, J. D. Guthrie, J. Neu, G. S. Chirayath, S. Huo*
Introducing an α‑Keto Ester Functional Group through Pt-Catalyzed Direct C−H Acylation with Ethyl Chlorooxoacetate.
ACS Omega 2020, 5, 8393−8402.
- B. J. Aguilar, Y. Zhao, H. Zhou, S. Huo, Y.-H. Chen, Qun. Lu*
Inhibition of Cdc42-intersectin interaction by small molecule ZCL367 impedes cancer cell cycle progression, proliferation, migration, and tumor growth.
Cancer Biology & Therapy. 2019, 20, 740-749.
- R. Mroz, D. A. K. Vezzu, B. Wallace, D. Ravindrananthan, J. Carroll, R. D. Pike, S. Huo*
A comparative study on phosphorescent cycloplatinated complexes based on tridentate C^N^N-coordinating ligands and phenylethynyl or phenyl ligand.
Chin. J. Org. Chem. 2018, 38, 171-182.
- D. C. McAteer, E. Javed, L. Huo, and S. Huo*
Platinum-catalyzed double acylation of 2-(aryloxy)pyridines via direct C-H activation
Org. Lett. 2017, 19, 1606-1609.
- J. Carroll, H. G. Woolard, R. Mroz, C. A. Nason, S. Huo*
Regiospecific acylation of cycloplatinated complexes. Scope, limitations, and mechanistic implications
Organometallics, 2016, 35, 1313-1322.
- Y. Li*, J. Carroll, B. Simpkins, D. Ravindranathan, C. M. Boyd, and S. Huo*
Computational and experimental study on selective sp2/sp3 or vinylic/aryl carbon-hydrogen bond activation by platinum(II): Geometries and relative stability of isomeric cycloplatinated compounds
Organometallics 2015, 34, 3303-3313. - D. A. K. Vezzu, Q. Lu, Y.-H. Chen,* and S. Huo*
Cytotoxicity of cyclometalated platinum complexes based on tridentate NCN and CNN-coordinating ligands: Remarkable coordination dependence
J. Inorg. Biochem. 2014, 134, 49-56. - J. Carroll, J. P. Gagnier, A. W. Garner, J. G. Moots, R. D. Pike, Y. Li, and S. Huo*
Reaction of N-isopropyl-N-phenyl-2,2’-bipyridin-6-amine with K2PtCl4: Selective C-H bond activation, C-N bond cleavage, and selective acylation
Organometallics 2013, 32, 4828-4836.
- C. F. Harris, D. A. K. Vezzu, L. Bartolotti, P. D. Boyle, and S. Huo*
Synthesis, structure, photophysics, and a DFT study of phosphorescent C*N^N- and C^N^N-coordinated platinum complexes
Inorg. Chem. 2013, 52, 11711-11722.
- S. Huo*, C. F. Harris, D. A. K. Vezzu, J. P. Gagnier, M. E. Smith, R. D. Pike, and Y. Li
Novel phosphorescent tetradentate bis-cyclometallated C^C*N^N-coordinated platinum complexes: Structure, photophysics, and a synthetic adventure
Polyhedron 2013, 52, 1030-1040. Werner special issue.
- C. F. Harris, D. Ravindranathan, and S. Huo*
Oxidative addition of heteroaromatic halides to Negishi reagent and subsequent cross-coupling reactions
Tetrahedron Lett. 2012, 53, 5389-5392.
- D. A.K. Vezzu, D. Ravindranathan, A.W. Garner, L. Bartolotti, M. E. Smith, P.D. Boyle, and S. Huo*
Highly luminescent tridentate N^C*N platinum(II) complexes featured in fused five-six-membered metallacycle and diminishing concentration quenching
Inorg. Chem. 2011, 50, 8261-8273.
- G. Zhang, L. Ding, R. Reneger, X. Wang, Q. Lu, S. Huo, and Y.-H. Chen*
Hydroxycamptothecin-loaded Fe3O4 nanoparticles induce human lung cancer cell apoptosis through caspase-8 pathway activation and disrupt tight junctions
Cancer Sci. 2011, 102, 1216-1222.
- A. W. Garner, C. F. Harris, D. A. K. Vezzu, R. D. Pike, and S. Huo*
Solvent-controlled switch of selectivity between sp2 and sp3 C-H bond activation by platinum (II)
Chem. Commun. 2011, 47, 1902-1904.
- J. C. Deaton*, R. H. Young, J. R. Lenhard, M. Rajeswaran, and S. Huo*
Photophysical properties of the series fac- and mer-(1-phenylisoquinolinato-N^C2¢)x(2-phenylpyridinato-N^C2¢)3-xIridium(III) (x = 1 to 3)
Inorg. Chem. 2010, 49, 9151-9161.
- D. Ravindranathan, D. A. K. Vezzu, L. Bartolotti, P. D. Boyle, and S. Huo*
Improvement in phosphorescence efficiency through tuning of coordination geometry of tridentate cyclometalated platinum (II) complexes
Inorg. Chem. 2010, 49, 8922-8928.
- D. A. K. Vezzu, J. C. Deaton, J. S. Jones, L. Bartolotti, C. F. Harris, A. P. Marchetti, M. Kondakova, R. D. Pike, and S. Huo*
Highly luminescent tetradentate bis-cyclometalated platinum complexes: Design, synthesis, structure, photophysics, and electroluminescence application
Inorg. Chem. 2010, 49, 5107-5119.
- D. A. K. Vezzu, J. C. Deaton, M. Shayeghi, Y. Li and S. Huo*
Acridinone/amine(carbazole)-based bipolar molecules: Efficient Hosts for fluorescent and phosphorescent emitters
Org. Lett. 2009, 11, 4310-4313.
- M. Rajeswaran, W. J. Begley, L. P. Olson, and S. Huo
Steric effects of substituted quinolines on lithium coordination geometry
Polyhedron 2007, 26, 3653-3660.
- J. C. Deaton, S. Huo, B. Lussier, C. T. Brown, J. C. Garnett, D. B. Blondell, and M. R. Landry
Vapor pressures of homo- and heteroleptic orthemetalated complexes of iridium
Digest of Technical Papers-Society for Information Display International Symposium, 2006, 37 (Bk. 1), 939-941.
- S. Huo,* J. C. Deaton, M. Rajeswaran, and W. C. Lenhart
Highly efficient, selective, and general method for the preparation of meridional homo- and heteroleptic tris-cyclometalated iridium complexes
Inorg. Chem. 2006, 45, 3155-3157.
- Z. Tan, B. Liang, S. Huo, J. Shi, E. Negishi
Zirconium-catalyzed asymmetric carboalumination (ZACA reaction) of 1,4-dienes
Tetrahedron: Asymmetry 2006, 17(4), 512-515.
- S. Huo*
Highly efficient, general procedure for the preparation of alkylzinc reagents from unactivated alkyl bromides and chlorides
Org. Lett. 2003, 5, 423-425.
- S. Huo, J. Shi, and E. Negishi
A new protocol for the enantioselective synthesis of methyl-substituted alkanols and their derivatives via hydroalumination-zirconium-catalyzed alkylalumination tandem process
Angew. Chem. Int. Ed. 2002, 41, 2141-2143. - T. Takahashi, M. Ishikawa, and S. Huo
Reaction of a double bond of zirconacyclopentadienes: Formation of 1,2,3,5-tetrasubstituted benzenes via the C-C bond cleavage
J. Am. Chem. Soc. 2002, 124, 388-389. - E. Negishi, S. Liu, C. Xu, and S. Huo
A novel, highly selective, and general methodology for the synthesis of 1,5-diene containing oligoisopenoids of all possible geometrical combinations exemplified by an efficient synthesis of coenzyme Q10
Org. Lett. 2002, 4, 261-264.
- S. Huo and E. Negishi
A convenient and asymmetric protocol for the synthesis of natural products containing chiral alkyl chains via Zr-catalyzed asymmetric carboalumination of alkenes. Synthesis of phytol and vitamins E and K
Org. Lett. 2001, 3, 3253. - K. Sato, Y. Nishihara, S. Huo, Z. Xi, and T. Takahashi
Preparation and reaction of monocyclic bis(cyclopentadienyl)titanacyclopentenes and –pentadienes
J. Organomet. Chem. 2001, 633, 18. - Takahashi, Y. Liu, C. Xi, and S. Huo
Grignard reagent mediated reaction of Cp2Zr(II)-ethylene complex with imines
Chem. Commun. 2001, 1(1), 31-32. - R. Hara, Y. Ura, S. Huo, K. Kasai, N. Suzuki, and T. Takahashi
Allene formation by coupling of propargylic ethers with olefins via b-alkoxide elimination of zirconacycle intermediates
Inorg. Chim. Acta 2000, 300-302, 741-748.
- Z. Xi, S. Huo, Y. Noguchi, and T. Takahashi
Formation of zirconacyclohexadienes from zirconacyclopentadienes and LiCHClSiR3
Chem. Lett. 2000, 218. - E. Negishi, S. Liu, C. Xu, and S. Huo
A general method for the synthesis of E and/or Z oligoisoprenoids based on Pd-catalyzed homoallyl-alkenyl and homopropargyl-alkenyl cross coupling and Zr-catalyzed carboalumination
Polyhedron 2000, 19, 592. - T. Takahashi, S. Huo, R. Hara, Y. Noguchi, K. Nakajima, and W. Sun
Reaction of zirconacyclopentadienes with CO in the presence of n-BuLi. Selective formation of cyclopentenone derivatives from two alkynes and CO
J. Am. Chem. Soc. 1999, 121, 1094. - B. Ji, Y. Shen, Y. Wu, K. Ding, and S. Huo
The molecular and crystal structure of an inclusion compound formed between acetylferrocence pyridine-2,6-diformylhydrazone and ethanol
Jiegou Huaxue 1998, 17, 319-324. - T Takahashi, Z. Xi, R. Fischer, S. Huo, C. Xi, and K. Nakajima
Intermolecular coupling reaction of alkynes with vinyl bromide with selective skeletal rearrangement
J. Am. Chem. Soc. 1997, 119, 4561. - C. Xi, S. Huo, T. Afifi, R. Hara, and T. Takahashi
Remarkable effect of copper chloride on diiodination of zirconacyclopentadienes
Tetrahedron Lett. 1997, 38, 4099. - T. Takahashi, Y. Nishihara, S. Huo, and M. Kotora
Highly regio- and distereoselective allylation and benzolation of zirconacyclopentane using copper salt
Chem. Commun. 1997, 1599-1600. - T. Takahashi, Z. Xi, Y. Nishihara, S. Huo, K. Kasai, K. Aoyagi, V. Denisor, and E. Negishi
Convenient preparative method of a, b-disubstituted cyclopentenone by zirconium promoted intermolecular coupling of an alkyne, EtMgBr or ethylene, and CO
Tetrahedron 1997, 53, 9123. - Y. Nishihara, T. Ishida, S. Huo, and T. Takahashi
Preparation and reactions of Cp2HfRCl, Cp2HfRR’ and hafnacyclopent-2-enes
J. Organomet. Chem. 1997, 547, 209-216. - T. Takahashi, R. Hara, S. Huo, Y. Ura, M. P. Leese, and N. Suzuki
Allene formation by the reaction of olefins with propargyl silyl ethers mediated by a zirconocene complex
Tetrahedron Lett. 1997, 38, 8723. - Y. Liu, Y. Wu, S. Huo, and H. Z. Yuan
Symmetrization reaction of mercurated ferrocenylimines. X-ray crystal structure of [Hg{(h5-C5H5)Fe(h5-C5H3CPh=NC6H4-4-Br)}2]
J. Organomet. Chem. 1997, 534, 7-13. - Y. Liu, Z. Yu, S. Huo, and Y. Wu
IR and chromatographic features of ferrocenylimines and their mercurated derivatives
Gaodeng Xuexiao Huaxue Xuebao 1996, 17, 901-905. - Y. Wu, S. Huo, and H. Yuan
Secondary interaction and n-p conjugation in ferrocenylimine derivatives of mercury as probed by Hg-199 resonance
Main Group Chemistry, 1996, 1, 253-256. - Y. Wu, S. Huo, L. Yang, and C. Du
Carbon-13 NMR spectra of cyclomercurated ferrocenylimines: Substituent effects and conformations in the ferrocenes
J. Organomet. Chem. 1995, 490, 249-254. - S. Huo, Y. Zhu, and Y. Wu
Crystal structures of anil of benzoylferrocene and its derivatives of mercury: Specific arrangement of the ferroceny and phenyl groups around the C=N bond
J. Organomet. Chem. 1995, 490, 243-247. - Y. Wu, S. Huo, and Y. Zhu
Crystal and molecular structure of [1-[(4-chlorophenyl)imino]ethyl]ferrocene and a structural comparison with Its cyclometallated derivatives
J. Organomet. Chem. 1995, 485, 161-164. - S. Huo, Y. Wu, X. Mao, and H. Yuan
Synthesis and mercuration of anils of benzoylferrocene
Tetrahedron 1994, 50, 10467-10476. - S. Huo, Y. Wu, C. Du, Y. Zhu, H. Yuan, and X. Mao
Synthesis, characterization and structure of cyclopalladated ferrocenylketimines
J. Organomet. Chem. 1994, 483, 139-146. - Y. Wu, S. Huo, Y. Zhu, and L. Yang
Studies on the cyclomercuration of [1-(arylimino)ethyl]ferrocenes and related structure-reactivity relationship
J. Organomet. Chem. 1994, 481, 235-242. - Y. Wu, S. Huo, and Z. Yu
Studies on the synthesis of heteroannularly substituted ferrocenesYuji Huaxue (Chinese J. Org. Chem.) 1994, 46, 38-43.
US Patents and Patent Applications
- US 6,835,835 B1
- US 6,824,895 B1
- US 7,029,766 B2
- US 7,417,146 B2
- US 7,476,739 B2
- US 5,517,984 B2
- US 7,553,556 B2
- US 7,579,090 B2
- US 7,597,967 B2
- US 7,718,276 B2
- US 7,736,756 B2
- US 7,767,316 B2
- US 7,842,406 B2
- US 2006134461 A1
- US 2006134459 A1