Research in the Burns Lab
Some Notes About Proteins
Proteins are responsible for catalyzing nearly all the necessary chemical reactions in biological systems. Often, they require metal ion cofactors to function properly, yet how proteins coordinate and control the reactivity of these species is a matter of intense research interest. Knowledge of metalloprotein structure is essential for developing concepts related to metal ion uptake and release, metal reactivity, protein-protein interactions, and regulatory processes. Hence, structural determination is essential for understanding the function of these biomolecules on a fundamental level. This is especially true if we wish to establish a basis for rational therapeutic intervention in disease pathways as there is a link between metal homeostasis and disease.
Many of the proteins I am most interested in are either unfolded or adopt non-globular structures under physiological conditions. These are referred to as ‘intrinsically disordered proteins’ or IDPs. Broad scale computational analyses of eukaryotic proteomes reveal that disorder is far from rare; two studies find an approximate 20% disorder content for eukaryotes (Cell. Mol. Life Sci. 2015, 72, 137-151; J. Mol. Biol. 2004, 337, 635-645). Understanding the connection between structural disorder and function is a new frontier in biochemistry; it requires new and refined methods for characterizing IDP structural ensembles and quantitative assessment of the balance of interactions that control the conformational distributions of IDPs.
Overlap exists between the protein classes discussed above which raises important questions: What types of metal binding motifs do IDPs have? Do they differ significantly from those of folded proteins? Interestingly, a number of metal-binding IDPS are implicated in disease, including Alzheimer’s disease (Abeta), Parkinson’s disease (alpha-synuclein), and Mad Cow’s disease (prion protein).
The Prion Protein
The prion protein (PrP) is responsible for a class of fatal neurological diseases called transmissible spongiform encephalopathies (TSEs). Divalent copper (Cu2+) is a natural ligand of PrP and binds to the highly dynamic N-terminal region. For the last several years we have been examining the mechanism of Cu2+ uptake by PrP as well as the thermodynamics of Cu2+ binding. Using both CD and Metal-Catalyzed Oxidation Mass Spectrometry we determined the number of binding modes used in the uptake of Cu2+ by the full metal binding-region spanning residues 57-98. In accordance with previous work, we find evidence for coordination of Cu2+ by multiple histidine imidazoles at low PrP:Cu2+ ratios. Further there appear to be several possible isomers of this coordination mode.
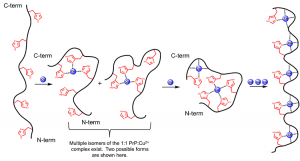
Proposed mechanism of Cu2+ uptake by the N-terminal domain of PrP
Our fluorescence spectroscopy studies have shown that Cu2+ and Zn2+ promote interactions between membrane-anchored peptides of the metal binding domain of PrP.
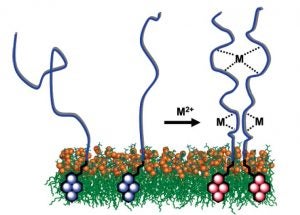
Copper and zinc promoted PrP-PrP interactions detected by fluorescence
Prothymosin-alpha
Prothymosin-alpha is an IDP located primarily in the nucleus of cells and consists of 110 amino acids. Its precise function is unknown; however, evidence suggests it is associated with cell proliferation and immunity. Prothymosin-alpha appears to specifically interact with Zn2+ and undergoes considerable structural rearrangement when exposed to this metal ion. Using mass spectrometry and dialysis in conjunction with atomic absorption spectrometry we show that the central segment of this protein, corresponding to residues 51-89, can bind up to five Zn2+ ions with moderate affinity. Our CD studies also demonstrate that this peptide becomes more compact as the temperature is raised indicating it undergoes thermally-induced collapse.
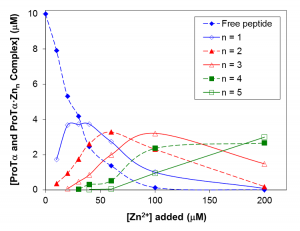
Concentration dependence of Zn2+ binding by prothymosin-alpha(50-89)N50W peptide determined by ESI MS
In collaboration with researchers at the Mt. Sinai School of Medicine we have explored the potent anti-HIV properties of prothymosin-alpha and peptides derived from it revealing how it mediates type I interferon induction.
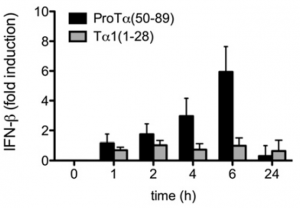
Interferon beta (INF β) mRNA induction over time in human macrophages by 5 μg/mL prothymosin-alpha(50-89) peptide (black bars)
15-deoxy, D12,14-prostamide J2 and Cancer
15-deoxy, Δ12,14-prostamide J2 (15d-PMJ2) is a novel product of the metabolism of arachidonoyl ethanolamide by COX-2 and preferentially induces cell death and apoptosis in tumorigenic nonmelanoma and melanoma cells.
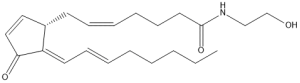
Chemical structure of 15d-PMJ2
Since 15d-PMJ2 does not kill nontumorigenic cells it displays selectivity which is a much sought after feature in potential chemotherapeutic agents. In pilot studies with melanoma tumor-bearing mice, animals treated with 15d-PMJ2 exhibited significantly reduced tumor growth and mean tumor weights compared with vehicle and untreated animals. In addition to synthesizing 15d-PMJ2 for in vitro and in vivo studies, we design and synthesize other prostaglandin and fatty acid derivatives to study their metabolism, mechanism of action, and structure-activity relationship. Ultimately, the goal is to develop therapeutics which can prevent and/or eliminate malignancies.